Successful ophthalmic drug delivery is a combination of pre-corneal retention time, corneal permeability, effectiveness of drug absorption, and dose-to-dose consistency. Whether the drug product is a suspension or microemulsion, it is a complex application involving careful analyses, research, and development efforts.
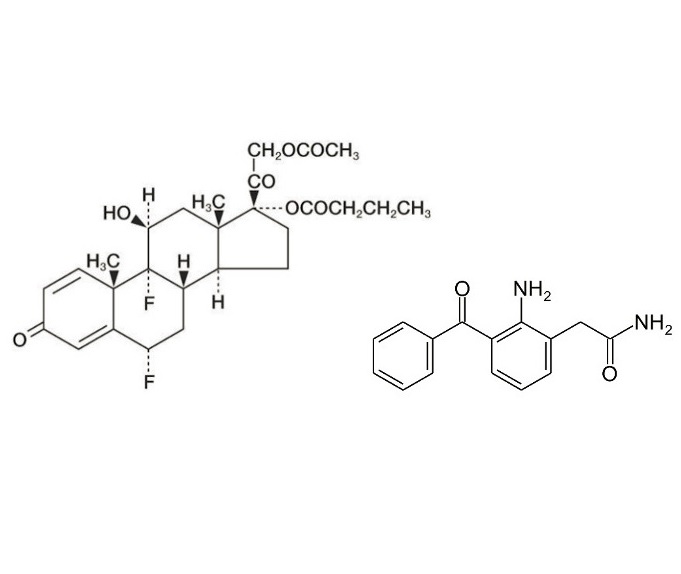
Physicochemical attributes such as particle size and particle size distribution, in particular, are two dominating factors that influence medicinal uptake and the extent at which the active pharmaceutical ingredient (API) reaches its intended segment of the eye. Difluprednate, a topical corticosteroid, and nepafenac, a nonsteroidal anti-inflammatory drug, both used for pain management post-cataract surgery, are no exception to this discussion. This note will examine how to accurately determine particle size and size distribution, repeatability, and reproducibility of these APIs to interpret shelf life and bioavailability alongside standards given by regulatory authorities.
Laser Scattering Particle Size Distribution Analyzer
Do you have any questions or requests? Use this form to contact our specialists.