
Figure 1: Structure and operating principle of a gas analyzer using FID.
Table of Contents
When hydrocarbons (HC) in the sample gas are flowed into the hydrogen flame, the hydrocarbons are oxidized, and an ionization reaction occurs. (Equation 1) Since the generated ions are proportional to the number of carbons, the gas concentration of hydrocarbons is measured by electrostatically collecting these ions and detecting them as an electric current.
\[CH \xrightarrow[(O)]{\text{Oxidation}} CHO^+ + e^-\]
Equation 1: Ionization reaction of hydrocarbons by hydrogen flame
A gas analyzer using FID continuously supplies fuel gas (hydrogen (H2) or a mixed gas of hydrogen and helium (He) or a mixed gas of hydrogen and nitrogen (N2)) and auxiliary gas (purified air) to produce a hydrogen flame. The sample gas is mixed with fuel gas and flowed into a high temperature hydrogen flame (>1500 K) at the tip of the jet nozzle, and the hydrocarbon molecules in the sample gas are oxidized and ionized. (Equation 1) These ions are collected by a collector electrode and detected as an electric current to measure the gas concentration of hydrocarbons. (Figure 1)
Figure 1: Structure and operating principle of a gas analyzer using FID.
A gas analyzer using FID is used to continuously measure the concentration of methane (CH4), non-methane hydrocarbons (NMHC)*1, and total hydrocarbons (THC)*2 in sample gas.
*1: NMHC: Abbreviation for Non-Methane Hydrocarbons, and general term for hydrocarbons other than methane.
*2: THC: Abbreviation for total hydrocarbons
The ppmC of a gas concentration unit is generally used in an FID gas analyzer. The ppmC (carbon equivalent concentration) is the gas concentration converted per a carbon, and ppmC is ppm multiplied by the number of carbons.
For example, if sample gas contains only propane (C3H8) 100 ppm as hydrocarbons, the FID analyzer measures 100 x 3 = 300 ppmC because propane (C3H8) has three carbons (C).
HORIBA uses a gas analyzer using FID to simultaneously and continuously measure the concentrations of methane (CH4), non-methane hydrocarbons (NMHC), and total hydrocarbons (THC), which are pollutants in the ambient air and exhaust gas. The structure and operating principle of the analyzer are explained here, taking as an example of an analyzer that continuously measures these hydrocarbon components in the ambient air.
An example of the overall structure of the analyzer is shown in Figure 2. The hydrogen flame of the FID ionizes most types of hydrocarbons in the sample gas. In order to measure gases, methane, non-methane hydrocarbons and total hydrocarbon in the sample gas, HORIBA's FID analyzer incorporates a non-methane remover unit using a selective combustion method (NMHC cutter) and a zero gas purifier.
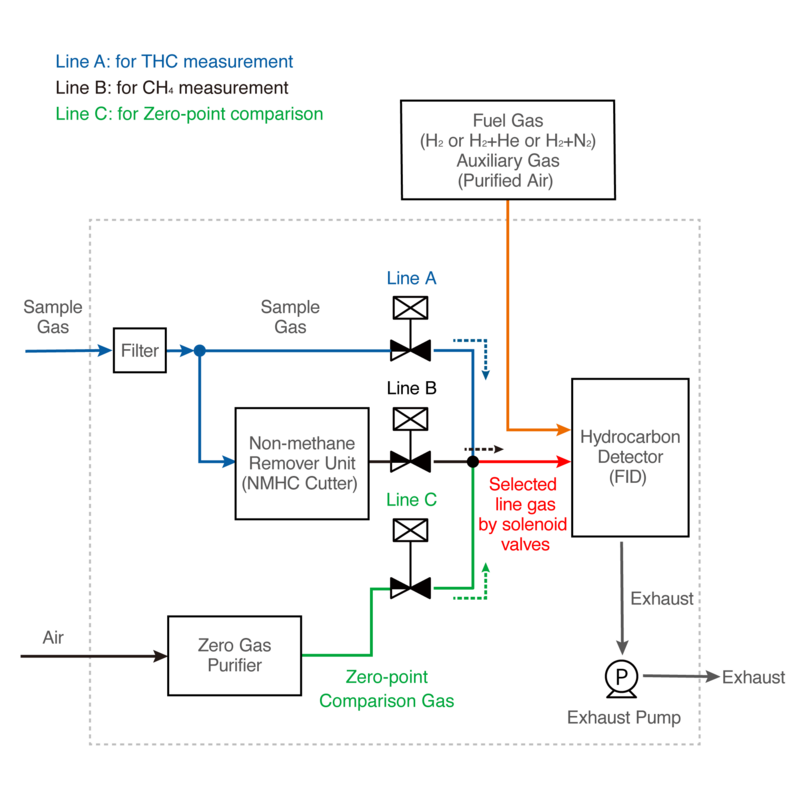
The line switching sequence is A, C, B, C, and so on.
Figure 2: Structure and operating principle of a total hydrocarbon (THC) gas analyzer
Hydrocarbons have different combustion temperatures depending on the number of carbons in their molecules. For example, the combustion temperature of propane is lower than methane. By controlling the combustion temperature according to this characteristic, all non-methane hydrocarbons in the sample gas be burned to generate a sample gas contains the only methane for hydrocarbons. This method is called the selective combustion method. In this process, it is important to control the combustion loss of methane in the sample gas.
The non-methane remover (NMHC cutter) in the analyzer utilizes this method.
The sample gas flows directly through the line A into a hydrocarbon detector with a built-in FID, and total hydrocarbons are measured. Next, solenoid valves switch to line C, and a hydrocarbon-free gas is flowed into the hydrocarbon detector (FID) as a zero-point comparison gas to reduce zero-point drift (*). Zero-point comparison gas is produced by removing moisture, hydrocarbons, etc. from the ambient air with a zero-gas purifier.
Then the solenoid valves switch to line B, methane-only gas is generated from the sampling gas by a NMHC cutter flows into the hydrocarbon detector (FID), and methane is measured. After methane measurement, switch to line C to flow zero-point comparison gas into the hydrocarbon detector (FID) to reduce zero-point drift (*).
Thus, selected gases are flowed into the hydrocarbon detector (FID) in the order of line A, C, B, and C.
Non-methane hydrocarbons are calculated from the difference in concentration between the total hydrocarbon and methane. (Lines A, B)
In addition, these gases are flowed into the same reaction cell and detected by the same detector by the solenoid valve switching function. This means that changes in sensitivity of the reaction cell and the detector over time, etc. are equally reflected in the detection of these gases, finally minimizing the difference in THC and CH4 sensitivity.
*)The zero-point drift is a shift of an analyzer's zero-point gradually in one direction due to temperature, aging, or other factors. The use of the comparison gas for a deviation of the zero point can reduce the influence of zero-point drift.
The FID gas analyzer measures methane, total hydrocarbons, and non-methane hydrocarbons based on measurement for an ionized known concentration of propane or methane by a hydrogen flame. For example, if an ionization rate of propane is the same for other hydrocarbons, FID can accurately measure any hydrocarbon in the sample gas, but if the ionization rate differs among hydrocarbons, the measurement will be influenced. The ionization rate varies depending on the structure of the hydrocarbon (e.g., double or triple bonds), the presence or absence of oxygen, and other factors.
Thus, measurement of the FID analyzer is influenced by the type and concentration of hydrocarbons contained in the sample gas. The FID analyzer requires mechanisms to ensure that the ionization rates of the hydrocarbons are the same as much as possible.
Depending on the concentration of oxygen, the hydrocarbon measurement is influenced. This is called oxygen interference.
For example, oxygen in sample gas causes some hydrocarbons to burn before ionization of the hydrocarbons, which changes the amount of ionization and influences the measurement.
HORIBA optimizes the following items to reduce the influence of differences in hydrocarbon ionization rates and the oxygen interference.
An FID analyzer is mainly used to measure hydrocarbons in the ambient air. The FID and NDIR analyzers measure hydrocarbons in exhaust gas according to the national and industrial environmental regulations. NDIR does not require utility gases such as fuel gas and FID can measure multiple gas components (THC, NMHC, CH4) simultaneously.
Hydrogen flame Ionization detection (FID) analyzer is active in the continuous measurement and monitoring of hydrocarbon emissions from mobilities and factories that lead to the formation of ozone (O3), a major component of photochemical smog, and the formation of harmful particulate matter. It is also used in the development of fuel efficiency of mobilities and engine combustion efficiency.
Table of Contents
Click here for a list of measurement principle explanations of continuous gas analyzers >
Do you have any questions or requests? Use this form to contact our specialists.


