Do you need fast, accurate results in your laboratory? Want an instrument that will grow with you and your business? The HORIBA Scientific ICP Spectrometers with their innovative design provide a tool that will increase your lab productivity not only with fast analysis time, but with less complicated methods, shorter warm-up time and better performance.
It was originally called ICP-AES for Inductively Coupled Plasma – Atomic Emission Spectrometry but since the introduction of ICP-MS, which is also an Atomic Emission Spectrometry technique, and since AES is dedicated to Auger Electron Spectroscopy, ICP-OES is preferred. ICP-OES is an elemental technique allowing the analysis of more than 70 elements of the periodic table.
As its name mentions, ICP-OES uses the Emission phenomenon for measurement. Samples are introduced into the system, and after several steps, atoms and ions of elements are excited.
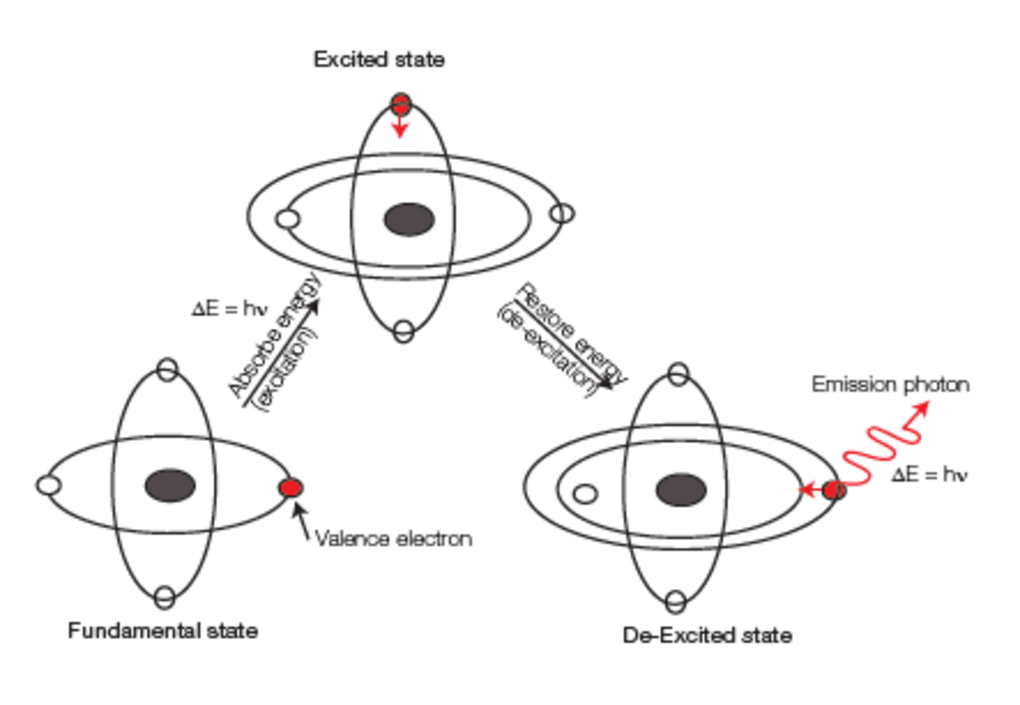
During de-excitation, atoms and ions of elements emit photons at characteristic wavelengths. Many photons of various intensities are emitted at the same time in all directions.
A plasma is defined as an ionized gas which is globally electrically neutral. To produce this ionized gas, an external source of energy is necessary, such as a spark, and the plasma is then sustained using an electrical field by means of an induction coil and a RF generator. The plasma can transfer a part of its energy to the sample so it can be atomized and eventually ionized.
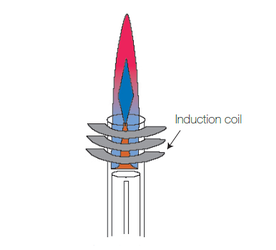
Fig.4: Inductively Coupled Plasma.
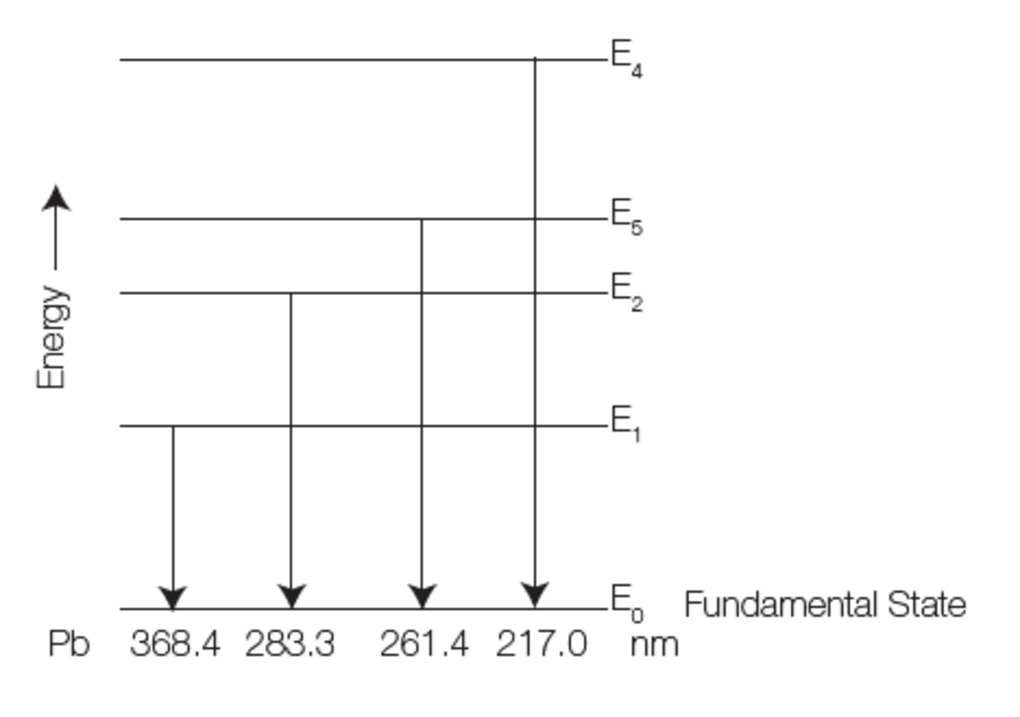
As high temperatures can be reached using a plasma, it is particularly suitable for emission spectrometry. The higher the temperature is, the more important the emission phenomenon is. Many elements emit photons and can then be analyzed.
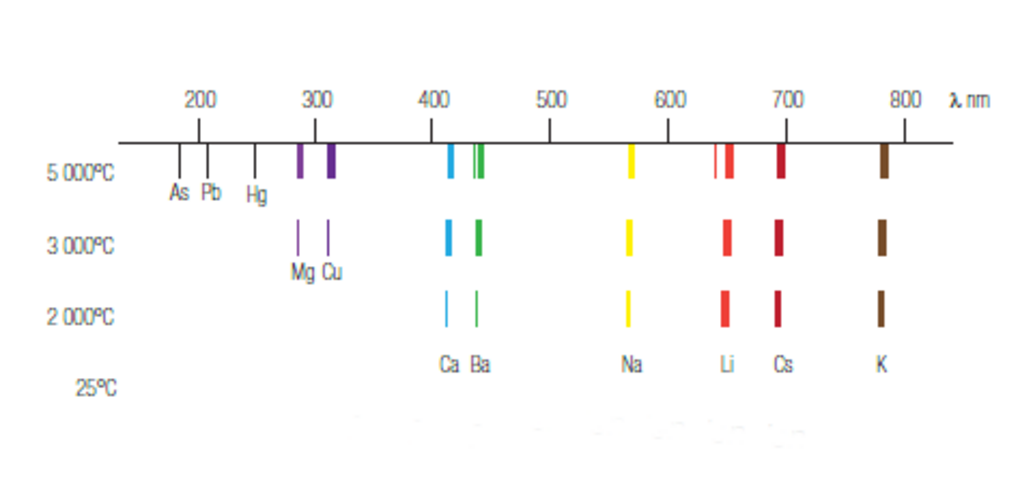
Fig.5: Effect of temperature on emission.
ICP-OES provides qualitative and quantitative information of elements present in a sample. Each line being characteristic of an element, the identification of several lines for a given element proves its presence.
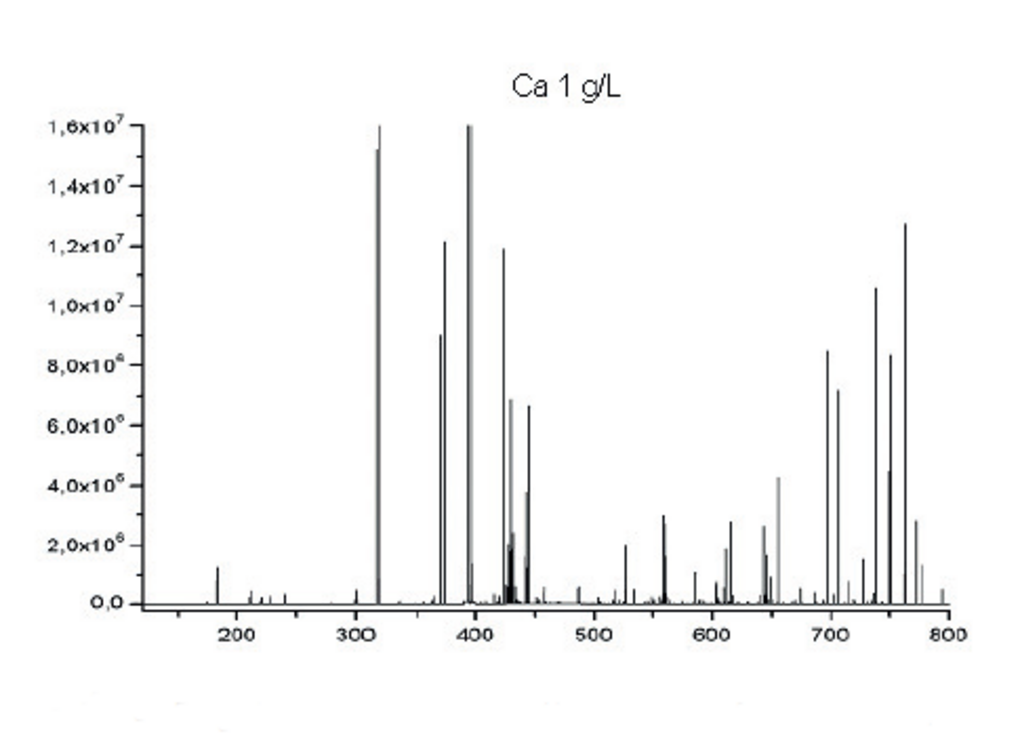
Fig.6: Emission spectrum for Ca (1g/L) on the full wavelength range of ICP-OES.
Quantitative information is provided by a calibration that should be done by the user before each analysis. Calibration may be different according to the application: External calibration, Standard addition, Matrix matching.
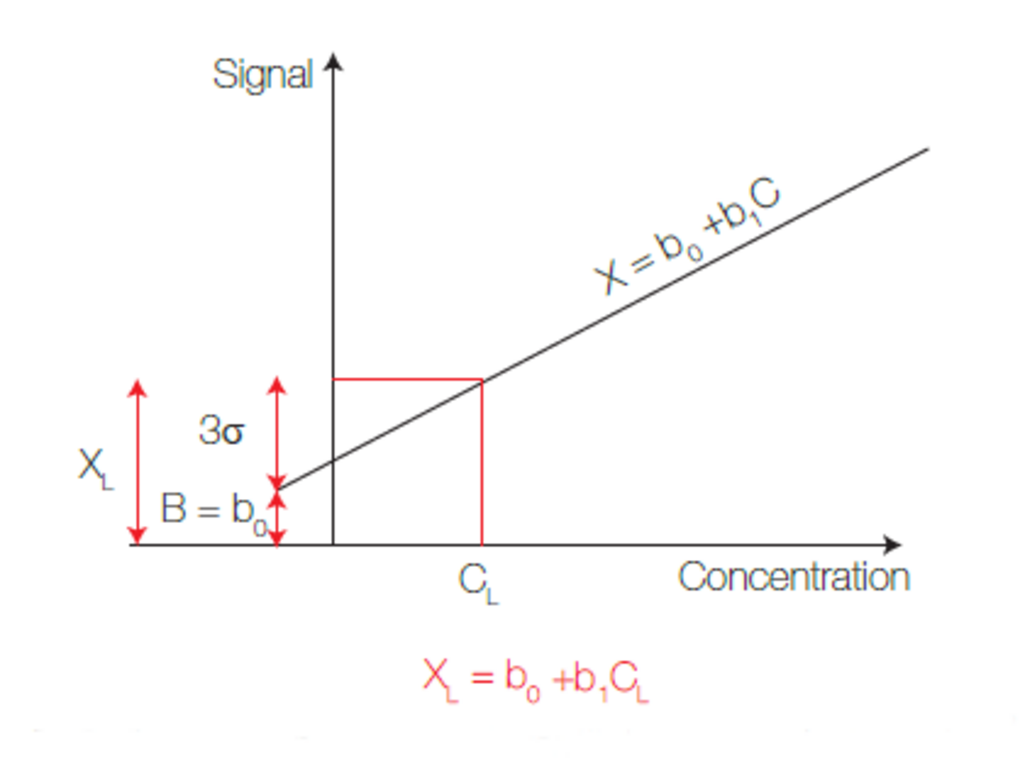
Fig.7: Calibration curve used for quantitative determination – Y-axis is for intensities and X-axis for concentrations. Least square are used for regression.
ICP-OES is a technique dedicated to liquid samples. Most of the time, samples are aqueous samples, water or solids after acid digestion (soils, steels, sludges…) or alkali flux preparation (geological samples, ceramics…). ICP-OES can also easily handle many organic matrices such as kerosene, xylene, white spirit, hexane, ethanol, ketones... This opens the way for the analysis of many compounds that are soluble in organic solvents (lubricating oils, edible oils, pharmaceutical compounds).
Solid samples can be analyzed by ICP-OES using special introduction accessories such as Spark Ablation (SPAB), Laser Ablation (LA) or Electro Thermal Vaporization (ETV). The choice of the accessory depends on the performances required and the material analyzed. All these accessories are destructive for the sample.
Spark Ablation (SPAB) provides analysis for conductive solids by a spark discharge. The solid is then transported to the plasma using an Argon flow. Calibration should be done using the same nature of materials as the sample, limiting the use of SPAB to metallurgical samples. Laser Ablation Laser Ablation (LA) is good at providing analysis for all kinds of solids, either conductive or non-conductive, and has the capability to focus on some parts of the area of the sample. Calibration should be done with materials similar to the sample (Certified Reference Materials-CRM) and actually, this step limits the use of LA to metallurgical samples or geological samples where many CRMs can be found.
Electro Thermal Vaporization provides solid analysis for all kinds of samples. The only requirement is that the sample should be sliced in small parts so as it can be introduced in a graphite tube. ETV can provide high sensitivity compared to SPAB or LA, and is used for analysis of traces in materials such as solar cells, and ultra high purity carbon. Calibration with ETV can be performed with water samples, simplifying the use of this accessory and expanding its capabilities.