Atomic absorption is a technique that allows the determination of a reduced number of elements, compared to ICP-OES. Fewer than 70 elements for flame atomic absorption and fewer than 45 elements for furnace atomic absorption can be determined.
Both furnace and flame atomic absorption are single element techniques with a limited dynamic range that is different for all elements. The analysis of several elements require several runs and several dilutions. This makes these techniques timeconsuming.
Flame atomic absorption is less sensitive than ICP-OES and it can achieve detection limits in the mg/L range. Only furnace atomic absorption can compete with the detection limits obtained with ICP-OES, but analysis time is much more important, up to 10 minutes per sample and per element.
Furnace atomic absorption can be used without any surveillance, like ICP-OES, as both techniques use inert gas (Argon or Nitrogen), allowing overnight analysis. For flame atomic absorption, flammable gas mixes, such as acetyleneair or acetylene-nitrous oxide, are used. The system cannot run without any surveillance so overnight analysis is basically impossible. Moreover, acetylene-nitrous oxide flame is difficult to manage as it may explode according to the ratio between the 2 gases.
ICP-MS which is a multi-element technique addressing as many elements as ICP-OES is using the capability of the plasma to create ions from the elements contained in the sample. Due to the high temperature in the plasma, the elements are ionized and ions are extracted from the plasma using a special interface. Ions are detected thanks to a mass analyzer according to their mass and their charges. The main interest of ICP-MS is that it can perform isotopic analysis and it can achieve very low detection limits typically at the ng/L level.
As a drawback, ICP-MS is not able to perform the analysis of high total dissolved solids samples as the cones of the interface may be blocked by deposits. Typically, ICP-MS has to be restricted to 0.2% (2 g/L) of dissolved solids, implying dilution of some samples.
Cost to use ICP-MS is also much more important than ICP-OES, as the system usually requires a clean room and ultra high purity reagents. To add to the operating cost, the detector has to be considered as a spare part because ions “exist” since they have a mass. The ions are transported into the detector and may also cause memory effects. More maintenance is then required with ICP-MS and particular attention has to be taken from one sample to another to avoid bias due to memory effects.
On the other hand, as photons have no mass, no memory effects are observed with ICP-OES, making it an easier technique to use that requires less maintenance.
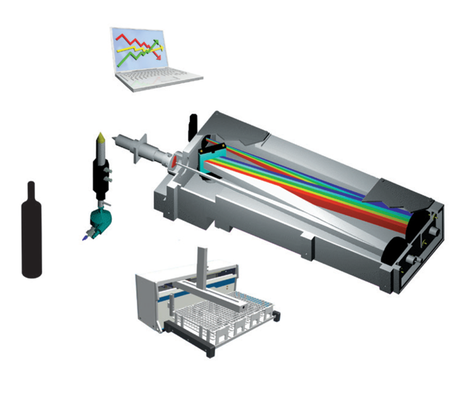
Optical Emission Spectrometry is the measurement of the light emitted by atoms and ions when de-excitation occurs. To allow selectivity of the system, i.e. the possibility to differentiate elements, a dispersive system is required to separate the wavelengths emitted by all the elements present in the sample.
According to the system, the ICP-OES will exhibit performances that can vary. After separation of wavelengths, a measurement device, i.e. the detector should be used. Different detectors, solid-state or photomultiplier tubes can be used according to the application and the performance required.