The nebulizer transforms the sample into an aerosol by mixing the liquid sample and Argon. Nebulization is mainly done using pneumatic nebulizers. For some applications, an ultrasonic nebulizer can be used. For pneumatic nebulizers, many systems exist and can be used according to the application, water analysis, samples with high content of dissolved salts, organics, volatile solvents…
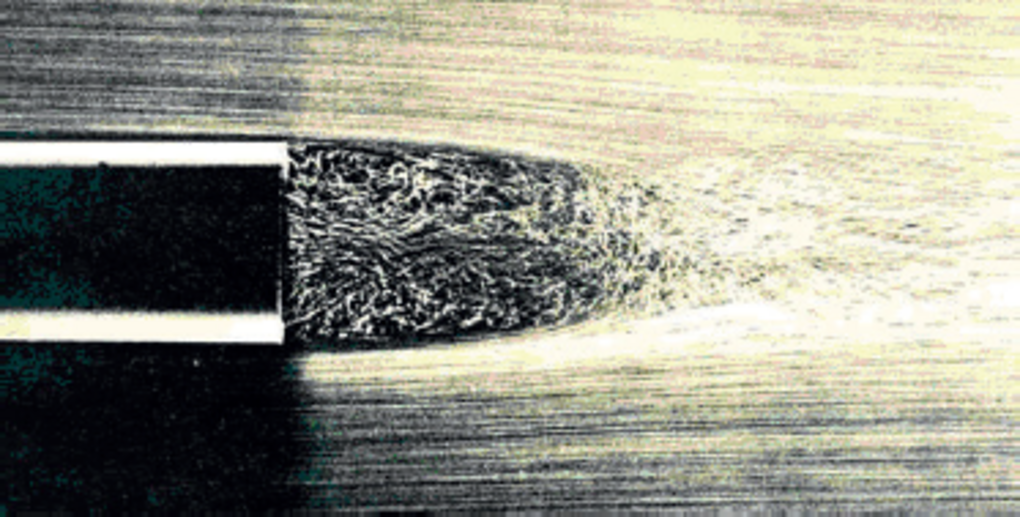
Fig.13: Creation of the aerosol at the gas-liquid interaction zone (end of the nebulizer).
Geometry of the nebulizer and particularly of its tip is critical as it will have a great influence on the quality of the aerosol produced. Aerosol droplet size produced by nebulizers is typically less than 100 μm. This aerosol is called a primary aerosol.
Fig.14: Typical drop diameter distribution after creation of the aerosol by the nebulizer (primary aerosol)
The spray chamber filters the aerosol created by the nebulizer. To ensure efficient energy transfer, the maximum size of droplets that enter the plasma is 10 μm. Filtration is done using different phenomena according to the design of the spray chamber.
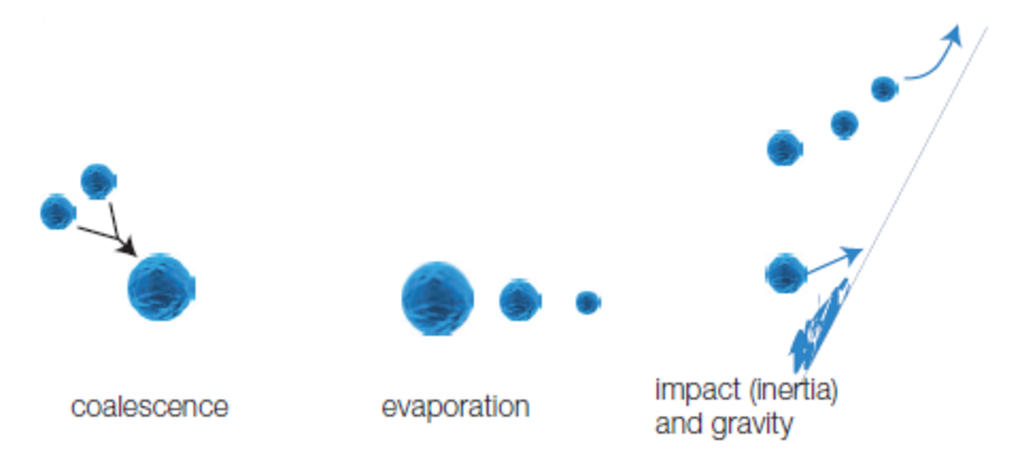
Fig.15: Phenomena involved in the spray chamber – Collision, coalescence, evaporation and impact.
Elimination of the biggest droplets can be done by gravity or centrifugal effect. Smaller droplets can be created by the evaporation effect or impact on walls of the spray chamber. This will be a competing effect with coalescence that will create bigger droplets from smaller droplets.
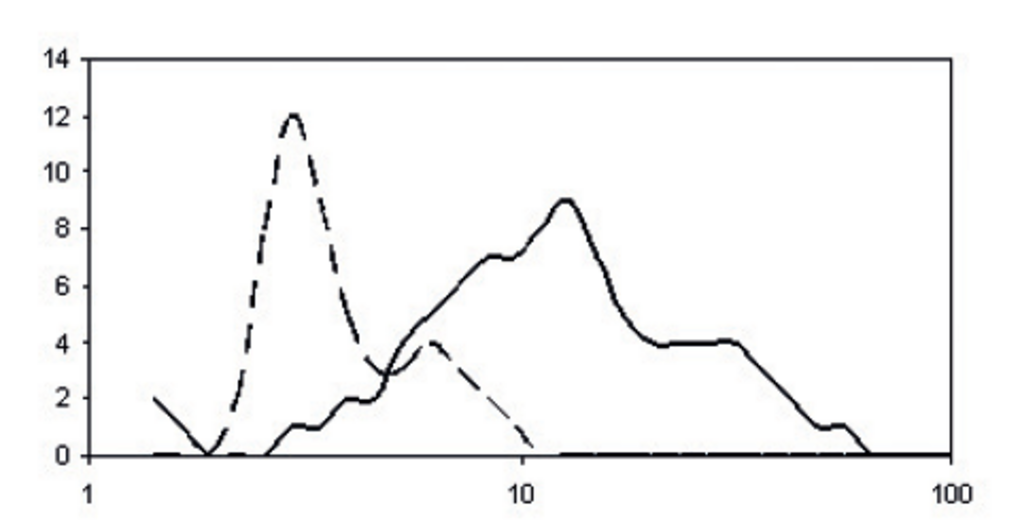
Fig.16: Typical drop diameter distribution after filtration in the spray chamber (tertiary aerosol).
Sheath gas is a device that is at the interface between the spray chamber and injector. This was originally patented by HORIBA Scientific. The role of sheath gas is to improve stability for matrices with a high content of dissolved solids.
To do it, a laminar flow of Argon is added after the spray chamber process. As the flow issued from the spray chamber is also a laminar flow, both spray chamber flow and sheath gas flow will never mix. Sample is then surrounded by dry Argon and has no contact with the walls of the injector. As there is no contact with the injector, memory effects are decreased and deposits on injector walls due to crystallization of salts will not occur.
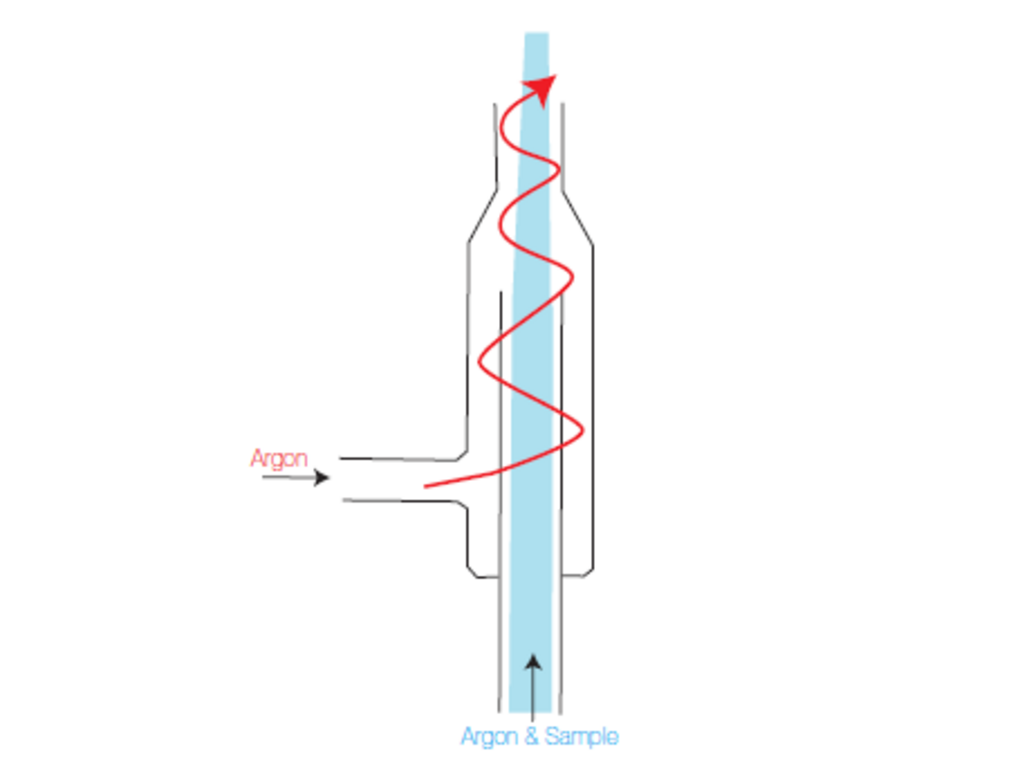
Fig. 17: Sheath gas device.
Sheath gas may also be used to improve sensitivity for alkali elements. Alkali elements are easily ionized in the plasma and to improve sensitivity, the temperature of the plasma should be reduced. Increasing the flow of sheath gas decreases the temperature of the plasma and improves sensitivity for alkali elements. Sheath gas flow is typically 0.2 L/min and can be increased up to 0.8 L/min for alkali determination.
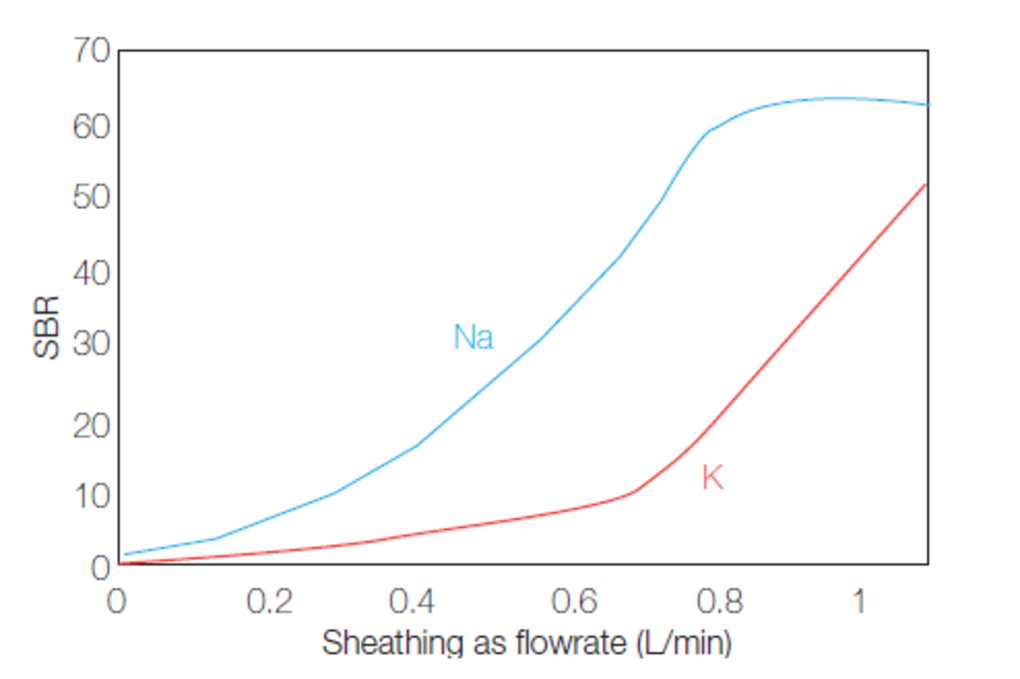
Fig. 18: Alkali elements signal improvement with sheath gas flow.
For matrices with high content of dissolved salts, some crystallization may occur in the nebulizer. To avoid clogging of the introduction system, an argon humidifier may be used. The aim of the argon humidifier is to saturate the argon used for nebulization with water. This has a lubricating effect, as well as a cooling effect at the tip of the nebulizer due to the restriction of diameter. With a lower temperature at the tip of the nebulizer and the lubricating effect, crystallization does not occur. HORIBA Scientific uses a unique membrane technology for the Argon humidifier, providing higher efficiency of saturation and improved performance, in addition to the absence of back-pressure in the system.
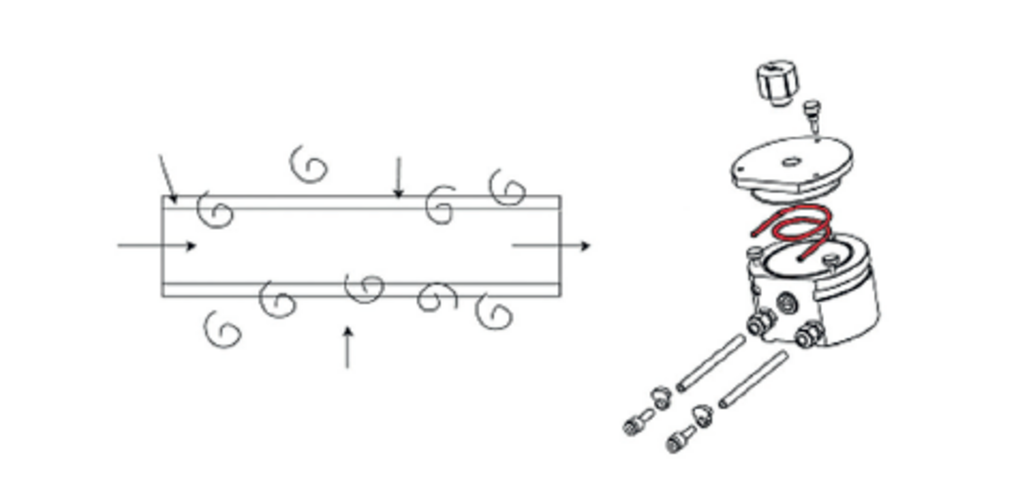
Fig. 19: Schematic of Argon humidifier membrane principle and of device with membrane in red.
The use of a peristaltic pump is mandatory to transport the sample to the nebulizer, even if the nebulizer can work using self-aspiration with the Venturi effect. Using the peristaltic pump allows to be independent of the viscosity of the sample. For some highly volatile solvents, self-aspiration has to be used but correction has to be done to compensate for the difference of volatility. The peristaltic pump will work with peristaltic pump tubing that should be selected according to the nature of the samples (aqueous, organics, ketones, etc.).
ICP-OES is a technique that can manage many samples and can be fully automated. For automated analysis, an autosampler may be used. Samples are placed on a tray and the autosampler will automatically go from one sample to another so as analysis can be performed.
Many nebulizers and spray chambers are available for ICPOES.
The main categories of nebulizers are:
The choice of the nebulizer is done according to the nature of the sample to ensure high stability and quality in the aerosol produced.
For spray chambers, main categories are:
The choice of the spray chamber is done according to the nature of the sample, and to the requirements, i.e. stability and sensitivity. The choice of the combination of nebulizer and spray chamber has to be done according to the application.
Applications can be divided into 5 main categories: